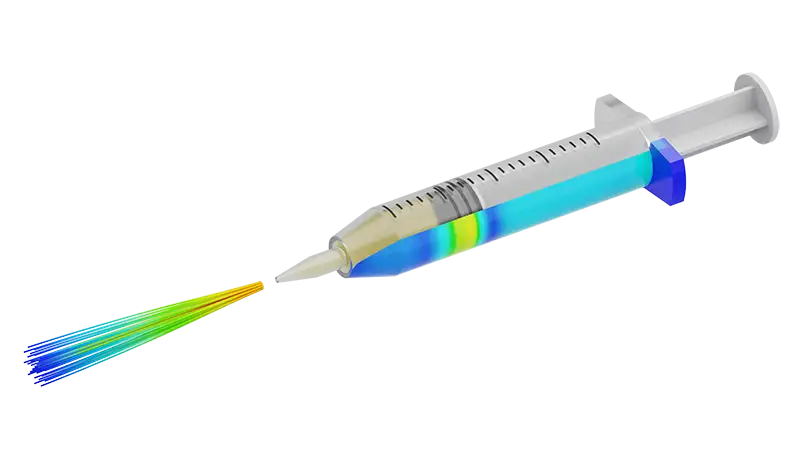
At PSN Labs, our integrated product development process is designed to take your device from concept to market with efficiency and precision. With an ISO 13485 certified engineering lab for the design and development of medical device components, we ensure that quality and compliance are built into every stage of the process. Our engineering services cover every step of product realization from early-stage design to final validation so your products meet both performance and regulatory requirements.
Our comprehensive approach to engineering centers on a process known as Integrated Product Development (IPD). Our process includes but is not limited to:
Throughout the process, we perform stage-gate reviews with clients and our internal teams, and maintain a complete design history file to support quality and compliance if required.
We combine rapid prototype iteration, virtual design cycles, rigorous testing to bring your vision to life. Our onsite functional prototyping leverages state-of-the-art capabilities, including:
Custom Test Method Development:
Our expert team creates methods for mechanical, electrical, environmental, packaging, and other critical testing parameters, ensuring that every aspect of device performance is evaluated.
Functional Device Testing & Novel Manufacturing Process Development:
We develop and optimize manufacturing processes that align with your unique product needs.
Method Validation & Transfer:
We ensure that our in-house test methods are seamlessly integrated into your workflows, with strict procedures to maintain data integrity and repeatability.
Design Verification Testing (DVT):
Combining benchtop testing with regulatory-grade simulation, we validate designs using both physical and virtual methods. Our collaborations with our ISO/IEC 17025:2017 accredited Test Lab allow us to offer both custom and standardized testing protocols.
We support your product development with cutting-edge computational tools and materials expertise:
Computational Modeling & Simulation (CM&S):
Our services include regulatory-grade model development for generating verification evidence for regulatory submittals. Tools in our simulation toolbox include physics and statistical modeling approaches such as: finite element analysis (FEA), computational fluid dynamics (CFD), other multiphysics, complete digital twins, and device-level statistical modeling.
Tolerance Analysis & Model Validation:
By predicting how variability in environmental conditions, dimensional variation, material and processing conditions, usage, shelf life, or sterilization affects performance, we ensure that our models understand your variable use and manufacturing environment.
Materials Engineering & Selection:
We go beyond datasheet specifications to evaluate the mechanical, chemical, and biocompatibility properties of materials. Our application-specific material selection process supports design for biocompatibility and all other aspects of material selection from the very start.
Proactive Design for Biocompatibility Strategy:
We embed biocompatibility considerations early in the development process to reduce late-stage testing risks and to set up long-term compliance strategies.
Our engineering services are built on a foundation of technical excellence and industry expertise. We offer:
Whether you are developing a new product or seeking to enhance an existing design, PSN Labs is your partner for creating innovative, reliable, and regulatory-ready solutions.
Contact us today to learn how our integrated engineering services can bring your next breakthrough to life.
Get in Touch