Vaporized Hydrogen Peroxide (VHP) can be an effective sterilization process for medical devices, surfaces, and healthcare facilities. The process uses hydrogen peroxide as a sterilant which is non-toxic and capable of permeating plastics, elastomers, and other materials that are permeable or semi-permeable.
Hydrogen peroxide is highly reactive. Hydrogen peroxide is a strong oxidizer. Hydrogen peroxide readily reacts with aldehydes, ketones, and hydrocarbons to form water (H2O)and carbon dioxide (CO2). An example of this reaction is formaldehyde (HCHO) and hydrogen peroxide (H2O2):
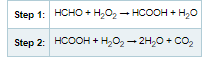
Formaldehyde is a substance of concern from a toxicological response perspective as it has chronic exposure concerns with respect to carcinogenicity. Therefore, the reduction and eradication of such substances of concern would reduce the bioburden and corresponding VOC profile for medical devices and building materials.
The VHP process includes three phases: Conditioning, sterilant exposure and post-conditioning, with all phases performed within a single chamber.
PSN Labs has experience in developing disinfection and sterilization cycles for medical devices. Material compatibility is generally positive – with most traditional metals and polymers being compatible. PSN Labs conducts material compatibility evaluations, feasibility studies, and can assist in development of sterilization cycles.
